Lisa Karosas
2016 Differentiated Project
QUESTION 4.2






Students Selected
Analogies were presented to all students attending synchronous instructional sessions and were, consequently, also available to those students who chose to learn asynchronously by watching recordings of these sessions at some other time.
Assessment data was collected for only 30 of these students. They were chosen according to the following criteria:
-
regular attendance in synchronous instructional sessions
-
active participation in synchronous instructional sessions
-
on-time completion of asynchronous assessments
In the virtual learning model, these behaviors typically result in average to high scores on assessments and general success with academic content, though this is not always the case. The demographics of this particular sample of students is as follows:
-
10 enrolled in the Honors level course
-
20 enrolled in the college-prep, "comprehensive" level course
-
5 possessing Individualized Education Plans (IEPs) to support special learning needs
-
33% earned an "A" (90-100%) for a final Semester 1 grade
-
23% earned a "B" (80-90%) for a final Semester 1 grade
-
23% earned a "C" (70-80%) for a final Semester 1 grade
-
6.7% earned a "D" (60-70%) for a final Semester 1 grade
-
6.7% earned an "F" (50-60%) for a final Semester 1 grade
Experimental Design

"Simple" Analogies, Standards
& Corresponding Assessment Questions
Dog Tug of War & Electronegativity
Makeovers & The Octet Rule
Standard-Based Assessment Questions
UNIT 2A: Atomic Structure
LESSON 2.10: The Bohr Atom
COURSE INFO
BIG
IDEA
ESSENTIAL QUESTION
STANDARDS
Atomic theory is the foundation for the study of chemistry.
CHEM.A.2.2
Describe the behavior
of electrons in atoms.
Standard-Based Assessment Questions
UNIT 2A: Atomic Structure
LESSON 2.12: Electron Orbitals
COURSE INFO
BIG
IDEA
ESSENTIAL QUESTION
STANDARDS
Atomic theory is the foundation for the study of chemistry.
3.2.C.A2
Compare the electron configurations for the first twenty elements of the periodic table.

Standard-Based Assessment Questions
UNIT 3A: The Periodic Table
LESSON 3.02: The Periodic Table
COURSE INFO
BIG
IDEA
ESSENTIAL QUESTION
STANDARDS
Periodic trends in the properties of atoms allow for the prediction of physical and chemical properties.
3.2.C.A1:
Explain the relationship between an element's position on the periodic table to its atomic number, ionization energy, atomic size, and classification of elements.
3.2.C.A2
Relate the position of an element on the periodic table to its electron configuration and compare its reactivity to the reactivity of other elements in the table.

Standard-Based Assessment Questions
UNIT 3A: The Periodic Table
LESSON 3.05: Periodic Trends
COURSE INFO
BIG
IDEA
ESSENTIAL QUESTION
STANDARDS
Atomic theory is the foundation for the study of chemistry.
3.2.C.A1:
Use electronegativity to explain the difference between polar and nonpolar covalent bonds.
CHEM.B.1.3.2:
Classify a bond as being either polar covalent, non-polar covalent, or ionic.
CHEM.B.1.3.3:
Use illustrations to predict the polarity of a molecule.
Standard-Based Assessment Questions
UNIT 4A: Chemical Bonding
LESSON 4.01: Monatomic Ions
COURSE INFO
BIG
IDEA
ESSENTIAL QUESTION
STANDARDS
Chemical bonding occurs as a result of attractive forces between atoms.
3.2.C.A2:
Relate the position of an element on the periodic table to its electron configuration and compare its reactivity to the reactivity of other elements in the table.
3.2.C.A2:
Explain how atoms combine to form compounds through both ionic and covalent bonding.








Square Dancing &
Double-Displacement Reactions
Princess and the Frog & Collision Theory
Cooking Recipes & Reaction Stoichiometry
Baseball & Ionic Bonding
Lady and the Tramp Spaghetti Scene
& Covalent Bonding
Legos & Synthesis/Decomposition Reactions
Vampire Love Triangles &
Single-Displacement Reactions
"Extended" Analogies, Standards
& Corresponding Assessment Questions
Hotels & Electron Behavior
Hotels & Electron Configurations
Calendars & Periodic Law
Dog Tug of War & Electronegativity
Makeovers & The Octet Rule
Baseball & Ionic Bonding
Lady and the Tramp Spaghetti Scene
& Covalent Bonding
Legos & Synthesis/Decomposition Reactions
Vampire Love Triangles &
Single-Displacement Reactions
Square Dancing &
Double-Displacement Reactions
Princess and the Frog & Collision Theory
Cooking Recipes & Reaction Stoichiometry

Standard-Based Assessment Questions
3.2.C.A2:
Explain how atoms combine to form compounds through both ionic and covalent bonding.
3.2.10.A2:
Compare and contrast different bond types that would result in the formation of molecules and compounds.
Chemical bonding occurs as a result of attractive forces between atoms.
STANDARDS
ESSENTIAL QUESTION
BIG
IDEA
COURSE INFO
UNIT 4A: Chemical Bonding
LESSON 4.03: The Ionic Bond
UNIT 4A: Chemical Bonding
LESSON 4.12: The Covalent Bond & Molecules
COURSE INFO
BIG
IDEA
ESSENTIAL QUESTION
STANDARDS
Chemical bonding occurs as a result of attractive forces between atoms.
Standard-Based Assessment Questions


Standard-Based Assessment Questions
3.2.C.A4:
Classify chemical reactions as synthesis (combination), decomposition, single displacement (replacement), double displacement, and combustion.
3.2.10.A4:
Describe chemical reactions in terms of atomic rearrangement and/or electron transfer.
CHEM.B.2.1.4:
Predict products of simple chemical reactions
Chemical reactions are predictable.
STANDARDS
ESSENTIAL QUESTION
BIG
IDEA
COURSE INFO
UNIT 5A: Chemical Reactions
LESSON 5.04: Synthesis Reactions
LESSON 5.05: Decomposition Reactions
COURSE INFO
BIG
IDEA
ESSENTIAL QUESTION
STANDARDS
Standard-Based Assessment Questions


Standard-Based Assessment Questions
STANDARDS
ESSENTIAL QUESTION
BIG
IDEA
COURSE INFO
COURSE INFO
BIG
IDEA
ESSENTIAL QUESTION
STANDARDS
Standard-Based Assessment Questions


Standard-Based Assessment Questions
3.2.C.A4:
Use stoichiometry to predict quantitative relationships in a chemical reaction.
3.2.10.A4:
Predict the amounts of products and reactants in a chemical reaction using mole relationships.
Chemical reactions are predictable.
STANDARDS
ESSENTIAL QUESTION
BIG
IDEA
COURSE INFO
UNIT 6A: Stoichiometry
LESSON 6.02: Mole-Number Relationships
Student Survey
Answers to the standard-based assessment questions were collected for students listed. These same students were surveyed to assess their perception of the effectiveness of this instructional technique. They were specifically asked to rank how they believed their ability to "remember" concepts and "understand" concepts was enhanced. They were also asked to indicate whether or not their interest in the lesson was affected. Finally, students were asked to rank each of the simple and extended analogies in this study according to each of these parameters. The complete survey administered is provided, below:






3.2.C.A2:
Explain how atoms combine to form compounds through both ionic and covalent bonding.
3.2.10.A2:
Compare and contrast different bond types that would result in the formation of molecules and compounds.
UNIT 5A: Chemical Reactions
LESSON 5.06: Single-Displacement Reactions
Chemical reactions are predictable.
3.2.C.A4:
Classify chemical reactions as synthesis (combination), decomposition, single displacement (replacement), double displacement, and combustion.
3.2.10.A4:
Describe chemical reactions in terms of atomic rearrangement and/or electron transfer.
CHEM.B.2.1.4:
Predict products of simple chemical reactions
UNIT 5A: Chemical Reactions
LESSON 5.06: Single-Displacement Reactions
Chemical reactions are predictable.
3.2.C.A4:
Classify chemical reactions as synthesis (combination), decomposition, single displacement (replacement), double displacement, and combustion.
3.2.10.A4:
Describe chemical reactions in terms of atomic rearrangement and/or electron transfer.
CHEM.B.2.1.4:
Predict products of simple chemical reactions
UNIT 5B: Chemical Kinetics
LESSON 5.06: Collision Theory
Chemical reactions are predictable.
3.2.10.A4:
Identify the factors that affect the rate of reaction.
CHEM.B.2.1:
Predict what happens during a chemical reaction.





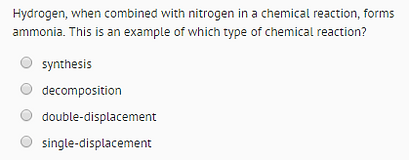








QUESTION 2.1
QUESTION 2.3
remember
understand
apply
QUESTION 2.25
QUESTION 3.19
remember
understand
QUESTION 3.5
QUESTION 3.12
evaluate
remember
QUESTION 3.19
QUESTION 4.23
apply
apply
QUESTION 4.1
QUESTION 4.18
apply
remember
remember
QUESTION 4.10
QUESTION 4.8
evaluate
evaluate
QUESTION 4.8
evaluate
QUESTION 4.8
QUESTION 5.8
evaluate
QUESTION 5.12
evaluate
understand
QUESTION 5.9
QUESTION 5.13
apply
evaluate
QUESTION 5.12
QUESTION 5.1
understand
evaluate
QUESTION 5.2
evaluate
QUESTION 5.7
QUESTION 6.2
evaluate
In what ways has the theory of the atom changed over time due to technological improvements?
In what ways has the theory of the atom changed over time due to technological improvements?
How does the distribution of electrons in atoms affect the formation of a compound?
How does the distribution of electrons in atoms affect the formation of a compound?
What factors determine the types of chemical bonds that form between particles?
What factors determine the types of chemical bonds that form between particles?
What factors determine the types of chemical bonds that form between particles?
What factors identify the types of chemical reactions?
What factors identify the types of chemical reactions?
What factors identify the types of chemical reactions?
According to the collision theory, what factors affect the rate of a chemical reaction?
How do stoichiometric ratios relate reactants to products in a chemical reaction?
In my classroom, I have routinely made informal references to relatable ideas in an effort to better connect with students' prior knowledge and initiate their exploration of a new concept. This year, as a professional goal, I set out to make these references more clear, more visual and more consistent; these references taught during the first two months of the year are considered to be "simple" because they are not mapped to show similarities and differences. I have examined the results of assessment questions relating to these analogies in an effort to compare them to those which were later developed as complete, "extended" analogies according to researched and documented best practices.
Assessment questions related to each standard-based analogy were chosen to represent as many levels of Bloom's taxonomy as possible; student performance was compared based on standards as well as rigor which should indicate whether or not depth of knowledge was influececed by this instructional strategy.
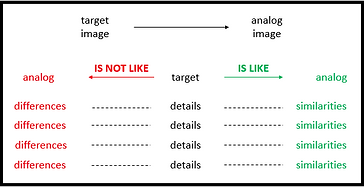
Upon initiation of this research, my standard practice of invoking "simple" analogies to enhance students' ability to connect to the content continued but was enhanced by the application of best practices wherein the targets and the analogs were mapped. In an effort to be as descriptive as possible and differentiate the content, I ensured that a visual map accompanied each analogy. Additionally, I adjusted the template for mapping the analogs as follows:
Assessment questions related to each standard-based analogy were chosen to represent as many levels of Bloom's taxonomy as possible; student performance was compared based on standards as well as rigor which should indicate whether or not depth of knowledge was influececed by this instructional strategy.